
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
- Complications
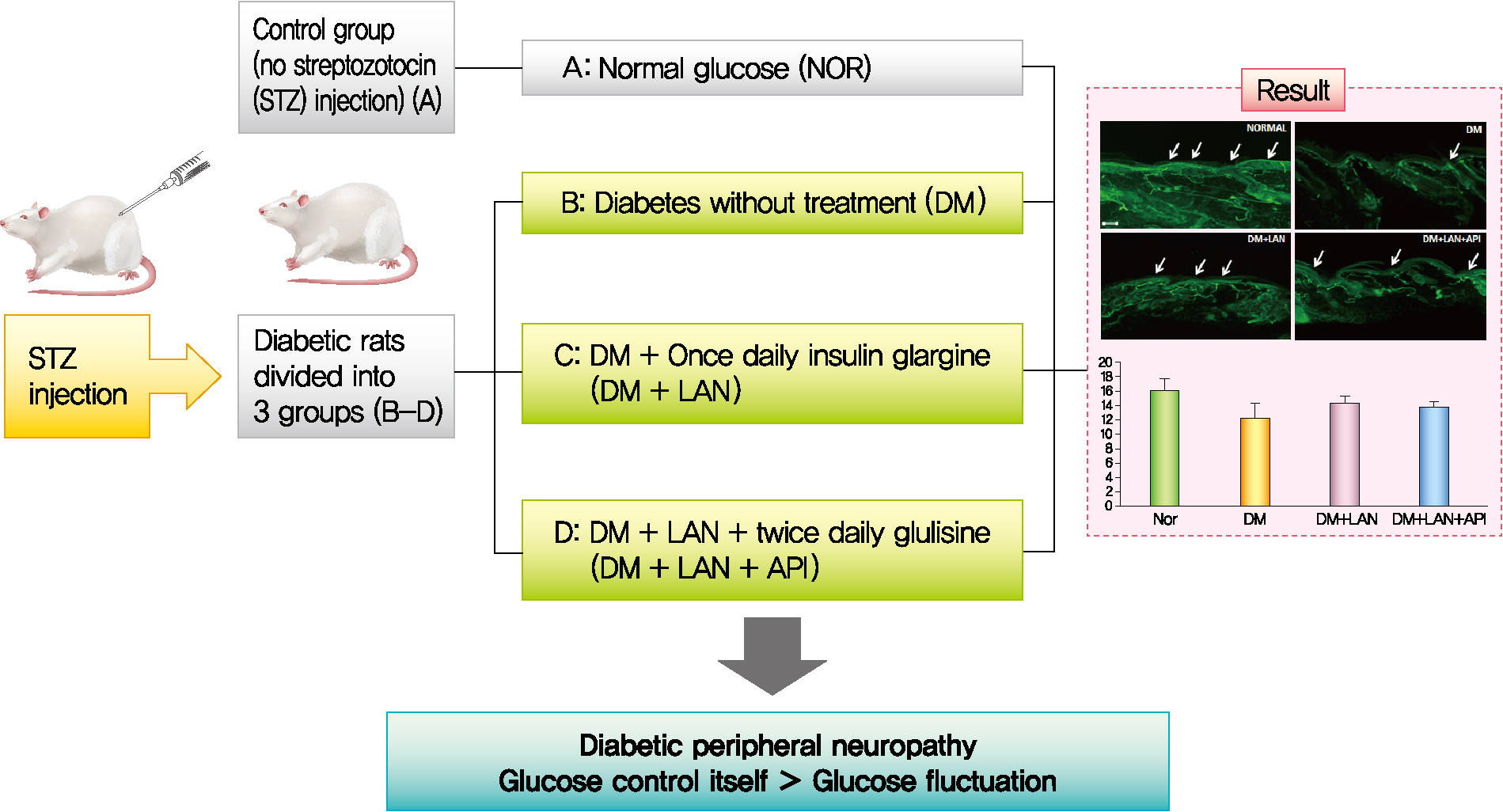
- Influence of Glucose Fluctuation on Peripheral Nerve Damage in Streptozotocin-Induced Diabetic Rats
- Yu Ji Kim, Na Young Lee, Kyung Ae Lee, Tae Sun Park, Heung Yong Jin
- Diabetes Metab J. 2022;46(1):117-128. Published online September 9, 2021
- DOI: https://doi.org/10.4093/dmj.2020.0275
- 5,248 View
- 179 Download
- 4 Web of Science
- 4 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub 
- Background
It is unclear whether glycemic variability (GV) is a risk factor for diabetic peripheral neuropathy (DPN), and whether control of GV is beneficial for DPN. The purpose of this study was to investigate the effect of GV on peripheral nerve damage by inducing glucose fluctuation in streptozotocin-induced diabetic rats.
Methods
Rats were divided into four groups: normal (normal glucose group [NOR]), diabetes without treatment (sustained severe hyperglycemia group; diabetes mellitus [DM]), diabetes+once daily insulin glargine (stable hyperglycemia group; DM+LAN), and diabetes+once daily insulin glargine with twice daily insulin glulisine (unstable glucose fluctuation group; DM+Lantus [LAN]+Apidra [API]). We measured anti-oxidant enzyme levels and behavioral responses against tactile, thermal, and pressure stimuli in the plasma of rats. We also performed a quantitative comparison of cutaneous and sciatic nerves according to glucose fluctuation.
Results
At week 24, intraepidermal nerve fiber density was less reduced in the insulin-administered groups compared to the DM group (P<0.05); however, a significant difference was not observed between the DM+LAN and DM+LAN+API groups irrespective of glucose fluctuation (P>0.05; 16.2±1.6, 12.4±2.0, 14.3±0.9, and 13.9±0.6 for NOR, DM, DM+LAN, and DM+LAN+API, respectively). The DM group exhibited significantly decreased glutathione levels compared to the insulin-administered groups (2.64±0.10 μmol/mL, DM+LAN; 1.93±0.0 μmol/mL, DM+LAN+API vs. 1.25±0.04 μmol/mL, DM; P<0.05).
Conclusion
Our study suggests that glucose control itself is more important than glucose fluctuation in the prevention of peripheral nerve damage, and intra-day glucose fluctuation has a limited effect on the progression of peripheral neuropathy in rats with diabetes. -
Citations
Citations to this article as recorded by- Glucose Fluctuation Inhibits Nrf2 Signaling Pathway in Hippocampal Tissues and Exacerbates Cognitive Impairment in Streptozotocin-Induced Diabetic Rats
Haiyan Chi, Yujing Sun, Peng Lin, Junyu Zhou, Jinbiao Zhang, Yachao Yang, Yun Qiao, Deshan Liu, Eusebio Chiefari
Journal of Diabetes Research.2024; 2024: 1. CrossRef - Artesunate Inhibits Apoptosis and Promotes Survival in Schwann Cells via the PI3K/AKT/mTOR Axis in Diabetic Peripheral Neuropathy
Xin Zhang, Zhifang Liang, Ying Zhou, Fang Wang, Shan Wei, Bing Tan, Yujie Guo
Biological and Pharmaceutical Bulletin.2023; 46(6): 764. CrossRef - The Potential of Glucose Treatment to Reduce Reactive Oxygen Species Production and Apoptosis of Inflamed Neural Cells In Vitro
Juin-Hong Cherng, Shu-Jen Chang, Hsin-Da Tsai, Chung-Fang Chun, Gang-Yi Fan, Kenneth Dean Reeves, King Hei Stanley Lam, Yung-Tsan Wu
Biomedicines.2023; 11(7): 1837. CrossRef - Relationship between acute glucose variability and cognitive decline in type 2 diabetes: A systematic review and meta-analysis
Haiyan Chi, Min Song, Jinbiao Zhang, Junyu Zhou, Deshan Liu, Victor Manuel Mendoza-Nuñez
PLOS ONE.2023; 18(9): e0289782. CrossRef
- Glucose Fluctuation Inhibits Nrf2 Signaling Pathway in Hippocampal Tissues and Exacerbates Cognitive Impairment in Streptozotocin-Induced Diabetic Rats
- Complications
- Effect of Empagliflozin, a Selective Sodium-Glucose Cotransporter 2 Inhibitor, on Kidney and Peripheral Nerves in Streptozotocin-Induced Diabetic Rats
- Kyung Ae Lee, Heung Yong Jin, Na Young Lee, Yu Ji Kim, Tae Sun Park
- Diabetes Metab J. 2018;42(4):338-342. Published online April 25, 2018
- DOI: https://doi.org/10.4093/dmj.2017.0095
- 4,000 View
- 64 Download
- 17 Web of Science
- 19 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader The effect of sodium-glucose cotransporter 2 inhibitors on peripheral nerves and kidneys in diabetes mellitus (DM) remains unexplored. Therefore, this study aimed to explore the effect of empagliflozin in diabetic rats. DM in rats was induced by streptozotocin injection, and diabetic rats were treated with empagliflozin 3 or 10 mg/kg. Following 24-week treatment, response thresholds to four different stimuli were tested and found to be lower in diabetic rats than in normal rats. Empagliflozin significantly prevented hypersensitivity (
P <0.05) and the loss of skin intraepidermal nerve fibers, and mesangial matrix expansion in diabetic rats. Results of this study demonstrate the potential therapeutic effects of empagliflozin for the treatment of diabetic peripheral neuropathy and nephropathy.-
Citations
Citations to this article as recorded by- Effect of empagliflozin in peripheral diabetic neuropathy of patients with type 2 diabetes mellitus
Sahar Mohamed El-Haggar, Yasser Mostafa Hafez, Amira Mohamed El Sharkawy, Maha Khalifa
Medicina Clínica.2024;[Epub] CrossRef - A Review of Recent Pharmacological Advances in the Management of Diabetes-Associated Peripheral Neuropathy
Osman Syed, Predrag Jancic, Nebojsa Nick Knezevic
Pharmaceuticals.2023; 16(6): 801. CrossRef - Renal intrinsic cells remodeling in diabetic kidney disease and the regulatory effects of SGLT2 Inhibitors
Wenwen Guo, Han Li, Yixuan Li, Wen Kong
Biomedicine & Pharmacotherapy.2023; 165: 115025. CrossRef - A systematic review on renal effects of SGLT2 inhibitors in rodent models of diabetic nephropathy
Aqsa Ashfaq, Myriam Meineck, Andrea Pautz, Ebru Arioglu-Inan, Julia Weinmann-Menke, Martin C. Michel
Pharmacology & Therapeutics.2023; 249: 108503. CrossRef - The impact of canagliflozin on the risk of neuropathy events: A post-hoc exploratory analysis of the CREDENCE trial
Jinlan Liao, Amy Kang, Chao Xia, Tamara Young, Gian Luca Di Tanna, Clare Arnott, Carol Pollock, Arun V. Krishnan, Rajiv Agarwal, George Bakris, David M. Charytan, Dick de Zeeuw, Hiddo J.L. Heerspink, Adeera Levin, Bruce Neal, David C. Wheeler, Hong Zhang,
Diabetes & Metabolism.2022; 48(4): 101331. CrossRef - Sodium Glucose Cotransporter-2 Inhibitor Protects Against Diabetic Neuropathy and Nephropathy in Modestly Controlled Type 2 Diabetes: Follow-Up Study
Fukashi Ishibashi, Aiko Kosaka, Mitra Tavakoli
Frontiers in Endocrinology.2022;[Epub] CrossRef - Protective effect of empagliflozin on gentamicin-induced acute renal injury via regulation of SIRT1/NF-κB signaling pathway
Sandy R. Botros, Asmaa I. Matouk, Aliaa Anter, Mohamed M.A. Khalifa, Gehan H. Heeba
Environmental Toxicology and Pharmacology.2022; 94: 103907. CrossRef - Empagliflozin mitigates type 2 diabetes-associated peripheral neuropathy: a glucose-independent effect through AMPK signaling
Noha F. Abdelkader, Marawan A. Elbaset, Passant E. Moustafa, Sherehan M. Ibrahim
Archives of Pharmacal Research.2022; 45(7): 475. CrossRef - Pathogenesis and Treatment of Diabetic Peripheral Neuropathy
Seon Mee Kang
The Journal of Korean Diabetes.2022; 23(4): 222. CrossRef - Empagliflozin and neohesperidin protect against methotrexate-induced renal toxicity via suppression of oxidative stress and inflammation in male rats
Adel T. Osman, Souty M.Z. Sharkawi, Mohamed I.A. Hassan, Amira M. Abo-youssef, Ramadan A.M. Hemeida
Food and Chemical Toxicology.2021; 155: 112406. CrossRef - Effect of exenatide on peripheral nerve excitability in type 2 diabetes
Tushar Issar, Natalie C.G. Kwai, Ann M. Poynten, Ria Arnold, Kerry-Lee Milner, Arun V. Krishnan
Clinical Neurophysiology.2021; 132(10): 2532. CrossRef - Effectiveness of Empagliflozin With Vitamin D Supplementation in Peripheral Neuropathy in Type 2 Diabetic Patients
Sanjana Mehta, Parminder Nain, Bimal K Agrawal, Rajinder P Singh, Jaspreet Kaur, Sabyasachi Maity, Aniruddha Bhattarcharjee, Jagannadha Peela, Shreya Nauhria, Samal Nauhria
Cureus.2021;[Epub] CrossRef - Targeting oxidative stress, proinflammatory cytokines, apoptosis and toll like receptor 4 by empagliflozin to ameliorate bleomycin-induced lung fibrosis
Ahmed M. Kabel, Remon S. Estfanous, Majed M. Alrobaian
Respiratory Physiology & Neurobiology.2020; 273: 103316. CrossRef - Empagliflozin reduces high glucose-induced oxidative stress and miR-21-dependent TRAF3IP2 induction and RECK suppression, and inhibits human renal proximal tubular epithelial cell migration and epithelial-to-mesenchymal transition
Nitin A. Das, Andrea J. Carpenter, Anthony Belenchia, Annayya R. Aroor, Makoto Noda, Ulrich Siebenlist, Bysani Chandrasekar, Vincent G. DeMarco
Cellular Signalling.2020; 68: 109506. CrossRef - Differential Effects of Empagliflozin on Microvascular Complications in Murine Models of Type 1 and Type 2 Diabetes
Stephanie A. Eid, Phillipe D. O’Brien, Lucy M. Hinder, John M. Hayes, Faye E. Mendelson, Hongyu Zhang, Lixia Zeng, Katharina Kretzler, Samanthi Narayanan, Steven F. Abcouwer, Frank C. Brosius, Subramaniam Pennathur, Masha G. Savelieff, Eva L. Feldman
Biology.2020; 9(11): 347. CrossRef - Pre-treatment with Empagliflozin ameliorates Cisplatin induced acute kidney injury by suppressing apoptosis
Maaly A. Abd Elmaaboud, Ahmed M. Kabel, Mohamed Elrashidy
Journal of Applied Biomedicine.2019; 17(1): 90. CrossRef - Effects of ticagrelor, empagliflozin and tamoxifen against experimentally-induced vascular reactivity defects in rats in vivo and in vitro
Yasmin Moustafa Ahmed, Basim Anwar Shehata Messiha, Mahmoud El-Sayed El-Daly, Ali Ahmed Abo-Saif
Pharmacological Reports.2019; 71(6): 1034. CrossRef - SGLT2 inhibition with empagliflozin attenuates myocardial oxidative stress and fibrosis in diabetic mice heart
Chenguang Li, Jie Zhang, Mei Xue, Xiaoyu Li, Fei Han, Xiangyang Liu, Linxin Xu, Yunhong Lu, Ying Cheng, Ting Li, Xiaochen Yu, Bei Sun, Liming Chen
Cardiovascular Diabetology.2019;[Epub] CrossRef - Empagliflozin Contributes to Polyuria via Regulation of Sodium Transporters and Water Channels in Diabetic Rat Kidneys
Sungjin Chung, Soojeong Kim, Mina Son, Minyoung Kim, Eun Sil Koh, Seok Joon Shin, Seung-Hyun Ko, Ho-Shik Kim
Frontiers in Physiology.2019;[Epub] CrossRef
- Effect of empagliflozin in peripheral diabetic neuropathy of patients with type 2 diabetes mellitus
- Complication
- Morphologic Comparison of Peripheral Nerves in Adipocyte Tissue from
db/db Diabetic versus Normal Mice - Kyung Ae Lee, Na Young Lee, Tae Sun Park, Heung Yong Jin
- Diabetes Metab J. 2018;42(2):169-172. Published online March 21, 2018
- DOI: https://doi.org/10.4093/dmj.2018.42.2.169
- 3,404 View
- 43 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Present study investigated the morphologic changes of autonomic nerves in the adipose tissue in diabetic animal model. Male obese type 2 diabetic
db/db mice and age matched non-diabeticdb/m control mice were used. Epididymal adipose tissue from diabeticdb/db mice with that from control heterozygousdb/m mice was compared using confocal microscopy-based method to visualize intact whole adipose tissue. Immunohistochemistry with tyrosine hydroxylase for sympathetic (SP), choline acetyltransferase for parasympathetic (PSP), and protein gene product 9.5 (PGP 9.5) for whole autonomic nerves was performed. The quantity of immunostained portion of SP, PSP, and PGP 9.5 stained nerve fibers showed decreased trend in diabetic group; however, the ratio of SP/PSP of adipose tissue was higher in diabetic group compared with control group as follows (0.70±0.30 vs. 0.95±0.25,P <0.05; normal vs. diabetic, respectively). Both SP and PSP nerve fibers were observed in white adipose tissue and PSP nerve fibers were suggested as more decreased in diabetes based on our observation.

 KDA
KDA
 First
First Prev
Prev





